Abstract
Introduction: The Philadelphia-negative myeloproliferative neoplasms are associated with chronic inflammation and accumulation of reactive oxygen species (ROS). Whole blood transcriptional profiling studies have most recently identified deregulation of several oxidative and anti-oxidative stress genes. Amongst the genes significantly downregulated is TP53 and the NFE2L2 or Nrf2 gene, the latter having a key role in the regulation of the oxidative stress response and in modulating both migration and retention of hematopoietic stem cells (HSCs). During MPN-disease progression, the HSC pool is steadily expanding with the egress of CD34+cell from stem cell niches into the circulation. In addition to Nrf2 several other genes are involved in this process, including CXCR4, which is also significantly downregulated in MPNs. Interferon-alpha2 (IFN) is recognized as a highly efficacious and promising agent in the treatment of MPNs. Taken into account that chronic inflammation with ROS accumulation is likely involved in the pathogenesis of MPNs, ultimately implying the induction of an altered redox balance of pivotal significance for stem cell mobilization in MPNs, we herein report the first study on the impact of IFN upon oxidative stress and anti-oxidative defence genes.
Methods: The HG-U133 Plus 2.0 microarray was used to profile expression of 38.500 genes in whole blood from ET (n=19), PV (n=41), PMF (n=9) and controls (n=21) (data set 1) and in ET (n=8), PV (n=21), and PMF (n=4) before and during treatment with IFN (data set 2). All patients received treatment with IFN, in the large majority in a dosage ranging from 45-90 ug x 1 sc/week. Total RNA was purified from whole blood amplified to biotin-labeled RNA, and hybridized to microarrays. The R statistical software was applied to perform data preprocessing and statistical analysis of microarray data.
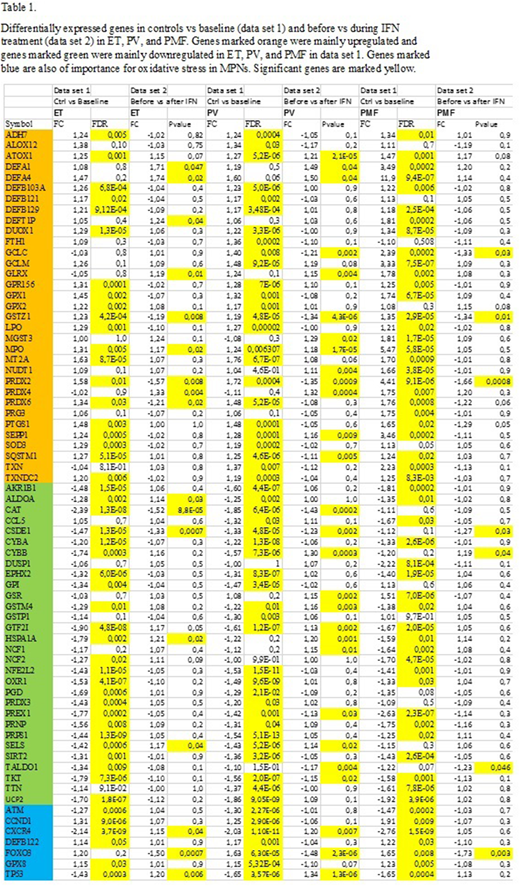
Results: Single gene analysis of 154 genes found to be included in previous studies focusing on deregulation of oxidative stress genes in various diseases were chosen for further analysis. In response to treatment with IFN, 19 genes were upregulated in ET including CXCR4 and TP53, and 11 genes were downregulated including FOXO3, PRDX2, and PRDX6. In patients with PV, ATOX1, CXCR4, SEPP1, and TP53 were among the 29 upregulated genes, and FOXO3 and PRDX2 were among the 14 downregulated genes. In response to treatment with IFN, 2 genes were upregulated in PMF which were CYBB and MSRA, and 9 genes were downregulated including PRDX2, and FOXO3 (all P<0.05). In a recent microarray study (data set 1), we have shown significant downregulation of genes associated with oxidative stress including ATM, CYBA, NRF2, PTGS1, SIRT2, TTN, and significant upregulation of AKR1B1, CCND1, DEFB122, GPX8 in ET, PV, and PMF vs controls. These genes were not significantly deregulated during IFN-treatment in any of the disease entities. Similarly, the significant downregulation of the NFR2 and the CXCR4 genes before IFN-treatment was no longer present after IFN treatment.
Discussion and Conclusions: The present study has shown IFN to have a major impact upon deregulated oxidative stress genes and antioxidative defence genes, implying a gene signature that mostly corresponds to decreased oxidative stress and enhancement of antioxidative defence genes. In regard to TP53 - being significantly downregulated before IFN treatment - this gene was significantly upregulated in ET and PV and no longer deregulated in PMF during IFN treatment. Downregulation of TP53 implies genomic instability due to an increased burden of oxidative stress upon the genome, which accordingly is reduced by IFN treatment. In addition to being the master regulator of the antioxidant response, Nrf2 also has a major role for normal stem cell function. Thus, our findings of a change in the Nrf2 gene expression from being significantly downregulated before IFN exposure to not being deregulated during treatment with IFN may imply an improvement in the antioxidant defence mechanisms against increased oxidative stress and accumulation of ROS. In conclusion, our findings of a major impact of IFN upon several oxidative stress genes and antioxidative defence genes may imply an IFN mediated dampening of genotoxic damage to hematopoietic cells and stromal cells as well, thereby ultimately diminishing the risk of additional mutations that might drive the malignant clone towards myelofibrotic and leukemic transformation.
Hasselbalch:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal